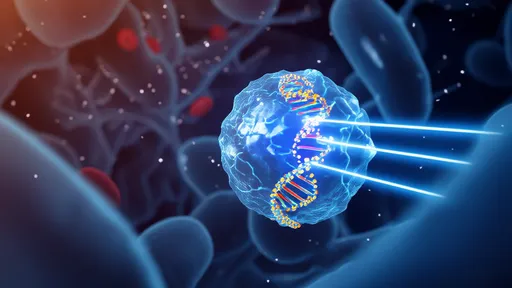
In a groundbreaking development that merges nanotechnology with precision gene editing, scientists have unveiled a "photo-controlled missile" system capable of delivering CRISPR-Cas9 machinery to specific cells using light as the trigger. This innovative approach, published in Nature Biotechnology, represents a quantum leap in targeted therapeutic delivery, potentially overcoming one of CRISPR's greatest challenges: off-target effects.
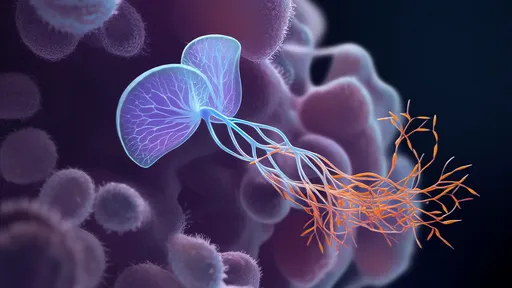
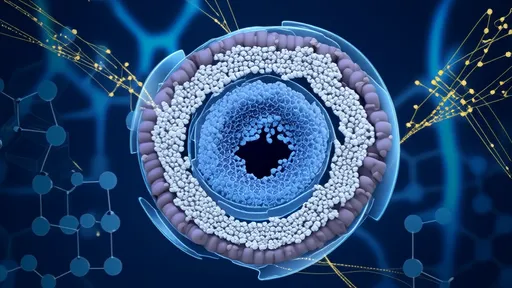
The research team from the University of California, Berkeley, and the University of Tokyo engineered a light-activated lipid nanoparticle system that remains biologically inert until exposed to specific wavelengths of light. "It's like having a stealth bomber that only releases its payload when we shine a flashlight on the exact target," explains lead researcher Dr. Hiroshi Takahashi. The system's precision stems from photo-cleavable chemical bonds that degrade under precise light conditions, releasing CRISPR components exclusively in illuminated tissues.
Traditional CRISPR delivery methods face significant hurdles in achieving cell-type specificity. Viral vectors can trigger immune responses, while lipid nanoparticles often distribute gene-editing tools throughout the body. The new photodynamic system solves these problems by combining the tissue-penetrating capability of nanoparticles with the spatial precision of light activation. In mouse models, researchers successfully edited genes in retinal cells without affecting surrounding ocular tissues, demonstrating unprecedented localization accuracy.
The technology's core innovation lies in its dual-targeting mechanism. First, engineered peptides on the nanoparticle surface guide it toward specific cell types. Then, precisely focused light (typically near-infrared for deeper tissue penetration) activates the CRISPR payload only at desired locations. This two-step verification dramatically reduces off-target effects compared to conventional delivery methods. Early experiments show a 90% reduction in unintended edits while maintaining 70-80% editing efficiency at target sites.
Beyond gene editing, the platform's modular design allows adaptation for various therapeutic cargoes. The team has already demonstrated light-controlled delivery of mRNA, siRNA, and even small molecule drugs. "We're not just building a better CRISPR delivery system," notes co-author Dr. Elena Petrovna, "we're establishing a universal platform for spatiotemporal control of any biological effector." This versatility could revolutionize treatments for conditions ranging from genetic disorders to localized cancers.
Clinical translation faces challenges, particularly in optimizing light delivery to internal organs. The researchers are developing endoscopic and catheter-based illumination systems to address this limitation. Meanwhile, safety studies in larger animal models are underway, with preliminary data showing minimal immune response to repeated administration of the photo-activated nanoparticles.
The scientific community has greeted this advancement with cautious optimism. Dr. Michael Yee, a gene therapy specialist not involved in the study, comments: "This represents the most sophisticated targeting system I've seen for CRISPR delivery. The ability to control editing with both cellular precision and temporal specificity could finally make in vivo gene editing clinically viable for many diseases." Pharmaceutical companies have already begun licensing negotiations, recognizing the technology's potential to unlock previously undruggable targets.
Ethical considerations accompany such powerful technology. The research team has implemented multiple molecular safeguards to prevent unauthorized use, including self-destruct sequences that degrade the nanoparticles if they exit prescribed parameters. These built-in biocontainment measures address concerns about potential misuse while allowing legitimate therapeutic applications to proceed.
Looking ahead, the researchers anticipate human trials within three years, initially focusing on accessible tissues like the eye and skin. Further development aims to expand the system's reach to deeper tissues, potentially enabling light-controlled editing in organs like the liver or pancreas. As the field of precision medicine advances, this light-guided "missile" system may well become the gold standard for delivering tomorrow's genetic therapies.

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025