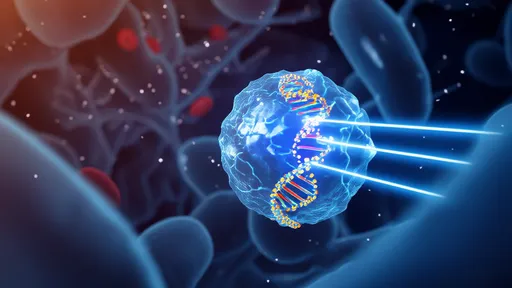
In a groundbreaking development that could revolutionize drug delivery to the brain, researchers have leveraged artificial intelligence to design protein carriers capable of crossing the blood-brain barrier (BBB). This biological fortress, which protects the brain from harmful substances while allowing essential nutrients to pass through, has long been a formidable obstacle in treating neurological disorders. The newly engineered "brain keys," as scientists call them, represent a quantum leap in targeted therapeutics.
The blood-brain barrier has been both a blessing and a curse for medical science. While it effectively shields the central nervous system from toxins and pathogens, this selective permeability also blocks approximately 98% of small-molecule drugs and nearly all large-molecule therapeutics from reaching their targets in the brain. For decades, researchers have sought ways to safely bypass this biological defense system to treat conditions ranging from Alzheimer's disease to brain tumors. Traditional approaches have included modifying drug chemistry or using invasive delivery methods, but these often come with significant limitations and risks.
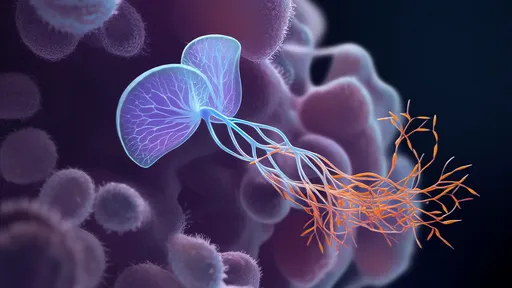
What makes this new approach remarkable is its biomimetic strategy. Instead of trying to force medications through the barrier or disrupt its integrity, scientists have turned to nature for inspiration. Certain proteins and peptides can naturally cross the BBB through receptor-mediated transcytosis—a process where molecules bind to specific receptors on the barrier's endothelial cells and get transported to the brain side. The research team used deep learning algorithms to analyze thousands of known BBB-shuttling proteins, identifying key structural and chemical features that enable this privileged passage.
The AI system, trained on extensive datasets of protein structures and BBB permeability measurements, generated novel protein designs optimized for brain delivery. These synthetic carriers incorporate multiple functional domains: one that binds to BBB transport receptors, another that protects the payload during circulation, and a third that releases the therapeutic cargo upon reaching the brain parenchyma. This modular design allows for customization based on the specific drug being delivered and the target neurological condition.
Early experimental results have been extraordinarily promising. In animal models, these AI-designed carriers demonstrated brain uptake levels several-fold higher than existing delivery methods, with minimal accumulation in peripheral tissues. Perhaps most exciting is their versatility—the same platform technology could potentially deliver antibodies, enzymes, gene therapies, or small molecules across the BBB. This opens up possibilities for treating previously inaccessible conditions like Parkinson's disease, glioblastoma, and rare genetic disorders affecting the central nervous system.
The development process represents a paradigm shift in biopharmaceutical design. Where traditional drug development might test hundreds or thousands of compounds through trial and error, this AI-driven approach uses generative models to create optimized candidates from first principles. The system can explore a vastly larger design space than human researchers, considering complex interactions between amino acid sequences, folding patterns, and surface chemistries that would be impossible to evaluate manually.
As the technology progresses, researchers anticipate it could dramatically shorten development timelines for neurological drugs while improving their efficacy and safety profiles. Current estimates suggest the first AI-designed BBB carriers could enter clinical trials within the next three to five years, potentially ushering in a new era of brain-targeted therapeutics. The implications extend beyond medicine—this work demonstrates how machine learning can accelerate scientific discovery by revealing patterns and solutions that elude conventional approaches.
Ethical considerations and safety monitoring will be paramount as this technology advances. While enhancing drug delivery to the brain offers tremendous therapeutic potential, it also requires careful control to prevent unintended consequences. The research team emphasizes that their carriers include built-in safeguards, such as tissue-specific activation mechanisms and clearance pathways, to minimize off-target effects. Regulatory agencies are already engaged in discussions about appropriate frameworks for evaluating these novel delivery platforms.
The convergence of structural biology, neuroscience, and artificial intelligence in this project highlights the transformative potential of interdisciplinary research. As our understanding of the blood-brain barrier's molecular machinery grows more sophisticated, so too do our tools for engaging with it therapeutically. These AI-designed protein carriers may well represent the first of many breakthroughs in overcoming biological barriers that have long limited medical progress.

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025