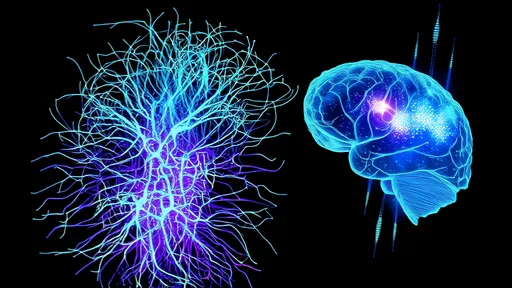
In a groundbreaking development for regenerative medicine, researchers have pioneered the use of hydrogel-based optical bridges to repair damaged optic nerves, offering new hope for patients with vision impairment. This innovative approach leverages the unique properties of biocompatible hydrogels combined with advanced optical technology to create a "neural light bridge" that can guide and stimulate nerve regeneration. The implications of this breakthrough extend far beyond ophthalmology, potentially revolutionizing treatments for various types of neural damage.
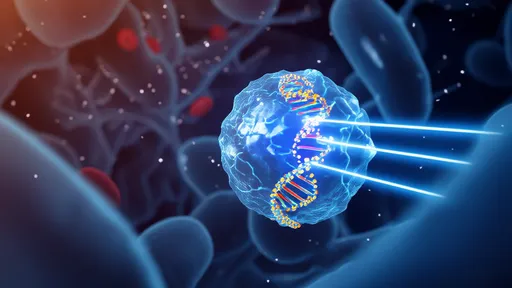
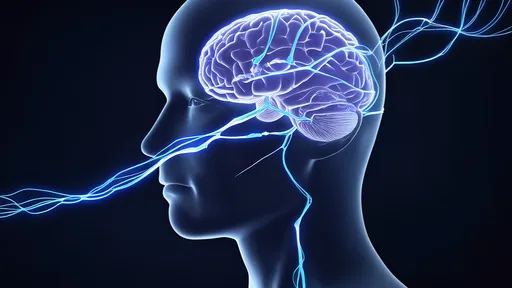
The human optic nerve, a crucial conduit for visual information between the eye and brain, has historically presented significant challenges for repair when damaged. Traditional approaches often yielded limited success due to the nerve's complex structure and poor regenerative capacity. However, the new hydrogel fiber technology acts as both a physical scaffold and optical conductor, creating an artificial microenvironment that promotes axonal growth while simultaneously delivering precise light-based stimulation to encourage neural reconnection.
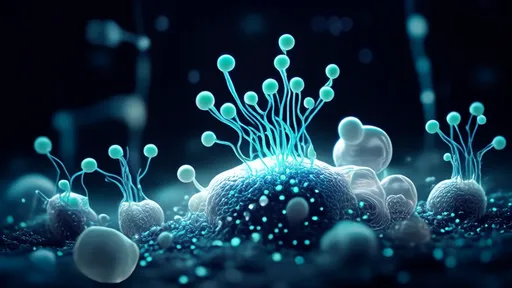
How the hydrogel optical bridge works represents a fascinating convergence of materials science and neurobiology. The specially engineered hydrogel fibers mimic the natural extracellular matrix while possessing exceptional light-guiding properties similar to conventional optical fibers. When implanted at the site of nerve damage, these translucent fibers provide structural support for growing nerve cells while transmitting specific wavelengths of light that appear to enhance cellular migration and axon extension. Early studies suggest the light stimulation may activate photosensitive ion channels in neural cells, potentially accelerating the regeneration process.
What makes this approach particularly remarkable is its dual functionality. The hydrogel scaffold not only bridges the physical gap in damaged tissue but also serves as a delivery system for optogenetic stimulation if needed. Researchers have demonstrated that incorporating light-sensitive proteins into regenerating neurons, combined with precise light delivery through the hydrogel fibers, can create an artificial signaling pathway during the critical healing period. This temporary bypass may help maintain neural connectivity until natural pathways are fully restored.
Clinical trials in animal models have yielded promising results, with restored visual function observed in subjects with previously severed optic nerves. The hydrogel fibers appear to reduce scar tissue formation while promoting directional nerve growth, addressing two major obstacles in neural repair. Importantly, the materials show excellent biocompatibility, gradually degrading as natural tissue regenerates without causing significant immune response or leaving harmful residues.
The potential applications of this technology extend well beyond optic nerve repair. Researchers speculate that similar hydrogel optical bridges could be adapted for spinal cord injuries or peripheral nerve damage, potentially helping patients regain mobility or sensation. The ability to combine structural support with optical stimulation opens new avenues for precisely controlling nerve regeneration processes that were previously impossible to guide with such accuracy.
While human trials remain on the horizon, the scientific community has greeted these developments with cautious optimism. The technology still faces challenges, including optimizing the light parameters for human physiology and ensuring long-term stability of the regenerated connections. However, the progress represents a significant leap forward in neural engineering, offering tangible hope that irreversible vision loss from optic nerve damage may someday become treatable.
This research exemplifies the growing trend of interdisciplinary solutions in medicine, where advances in material science, optics, and biology converge to solve previously intractable medical problems. As development continues, the neural light bridge approach may fundamentally change our understanding of nerve regeneration and open new possibilities for restoring function after various types of neural injuries.

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025